Nutritional management (both short and long term) based on continuous nutritional assessment is of prime importance in the management of major burn injury with more than 20% Total Body Surface Area (TBSA) burns.
Why so? Since in major burns, there is—
- an acute massive fluid shift initially,
- a prolonged hypermetabolic (catabolic) state following it— From 24-72 hours up to ~2 years post-burn
- an increased risk of loss of ‘lean body mass‘ (LBM) and
- an increased free radical production.
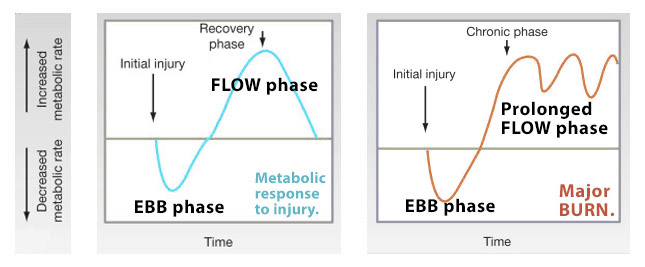
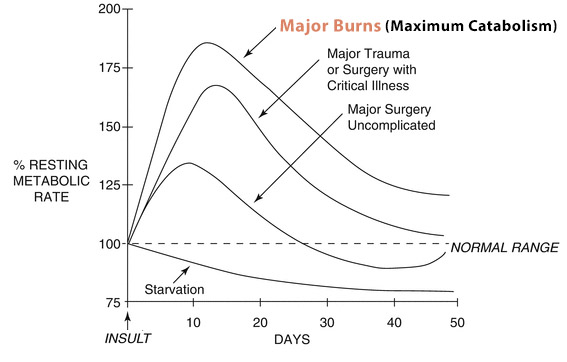
On one hand, insufficient intake of calories and protein can lead to lead to loss of ‘lean body mass’ (LBM), which can induce:
- immune dysfunction (at 10% loss of LBM)- higher chance of infection,
- impaired wound healing (at 20% loss of LBM),
- increased mortality (at 40% loss of LBM);

On the other hand, overfeeding can have equally serious complications due to fat accretion (gradual excess production):
- hyperglycemia,
- fatty liver infiltration,
- hypercapnia and metabolic acidosis,
- hypertriglyceridemia,
- azotemia,
- prolonged ventilator dependency.
Understanding the physiologic response to burn stress and adequate nutritional management is crucial in the recovery of patients with major burns.
Here we won’t be discussing fluid requirements but only calorie, protein, and other nutritional requirements.
Timing, Type, Route
Goal in Major Burn should be: Enteral nutrition through nasojejunal (N-J) tube by 48 hours
TIMING: Start by 48 hours gradually so that full nutritional goal is achieved by 72 hours.
TYPE: Enteral nutrition through nasojejunal (N-J) tube – is the route of choice universally in large areas of burn, as— it maintains gut integrity, supports the immune system and generally well tolerated.
For the conscious, motivated patient with an intact appetite and swallowing function and not severe/extensive burns (40%), direct oral intake is the preferred feeding modality. In motivated patients, supplemental “protein shakes” are useful is boosting protein-calorie nutrition and avoiding N-J tube. But on close monitoring, if the patient fails to take in adequate calories due to decreased appetite, depressed mental status, pain, or any other practical obstacle to eating. If a patient consistently fails to meet estimated caloric needs with a PO diet, supplemental feeds via naso-enteric tube should be initiated.
Parenteral nutrition should be completely avoided in burns and is currently used as “bridge” or “rescue” therapy only when enteral nutrition may not be possible for more than 7 days due to intolerance (diarrhoea, distension), frequent surgeries or due to technical problems.
ROUTE: Nasoenteric (jejunal) feeds are preferred as gastric ileus is common in burns patients. Compared to nasogastric feeds, nasoenteric feeds are better tolerated and not interrupted by surgeries, but it doesn’t decrease the risk of aspiration.
Total Energy requirement (Caloric Goal, Caloric estimation)
Factors affecting energy consumption:
- size and depth of burns,
- presence of inhalation injury,
- burn wound closure and surgical procedures,
- physical therapy,
- sepsis,
- ambient temperature,
- certain medications.
The two most common methods of calorie estimation are- i) Indirect calorimetry, ii) Mathematical calculations.
i) Indirect calorimetry (IDC)
It’s a respiratory test that measures actual REE (Respiratory Energy Expenditure) by measuring the exchange of O2 and CO2 by the lungs. It provides energy expenditure based on the test time frame (typically at rest) and thus needs to be multiplied by 1.2 to 1.3 allow for variability and activity.
It’s accurate and the “gold standard” but not always practical as it’s very expensive for the patient and the clinic (to acquire the machine and trained operating staff). However, equipment for bedside performance of IDC has become more accessible over the last decade, and the associated technology has improved ease of use.
ii) Mathematical calculations
This is the more practical way of calculating calorie requirement although it’s less accurate (as the burns patient have highly variable energy expenditure) and may lead to underfeeding and also overfeeding.
Various mathematical equations for requred kcal/day are mentioned below:
Adults:
1. Harris-Benedict Equation (1919)
- Men: 66.5 + 13.8 x Wt.(kg.) + 5 x Ht.(cm.) – 6.76 x Age(yrs.)
- Women: 655*+ 9.6 x Wt.(kg.) + 1.86 x Ht.(cm.) – 4.68 x Age(yrs.)
* 💡 655 is correct- not a mistake in decimal point 🙂
This estimates BEE (Basal Energy Expenditure). Multiply the result with “Stress Factor” of 1.2 to 1.5 to get the final energy requirement.
2. Calories per Kilogram- Dickerson et al. (2002)
- Non-obese: 25 to 35 kcal/ kg B.W.
- Obese / critically-ill: 21 kcal/ kg B.W.
3. Curreri (1974)- Grossly overestimates, rarely used now.
25 x Wt.(kg.) + 40 x %TBSA
Rarely used now, due to marked tendency to over-estimate.
Pediatric:
1. DRI- Dietary Reference Index (2010)- previously called RDA*.
- Online calculators: Google the term”DRI calculator”.
- IOS/Android apps: https://digital.gov/2015/02/05/focus-on-nutrition-with-dri-calculator-for-healthcare-professionals-app/
It takes into account: Age, Sex, Height, Weight and Activity level (Sedentary, Low active, Active, Very active)
*RDA- Recommended Dietary Allowances
2. Galveston (1989- 1993)- Overestimates.
0–1 yrs: 2100 kcal/m2 BSA + 1000 kcal/m2 BSAB
1–11 yrs: 1800 kcal/m2 BSA + 1300 kcal/m2 BSAB
> 12 yrs: 1500 kcal/m2 BSA + 1500 kcal/m2 BSAB
** It’s difficult to calculate as it requires BSA and BSA Burnt (BSAB) in m2.
3. Curreri Junior (1986)- Grossly overestimates, rarely used now.
RDA + 15, 20 and 25 x %TBSA for age groups <1, 1-3 and 4-15 years respectively.
Diet Composition
Once the ‘Caloric Goal’ is set, the ratio of Carbohydrate, Protein, and Fat has to be considered for optimal nutritional support. In burns, high carbohydrate-protein diet is required.
Carbohydrates
Carbohydrates should be the prime energy source as they may limit the loss of lean body mass (LBM) by preventing additional compensatory protein catabolism.
Like in non-burned individuals, 50 – 60% of the ‘caloric goal’ should come from carbohydrates. Though there is an increased caloric demand, the human body has a finite capacity for glucose oxidation which is approximately 5 g/kg/day in adult and 7 g/kg/day in pediatric burn patients and any carbohydrate (glucose) provided in excess of these will lead to hyperglycemia and ultimately lactic acidosis, increase fat production (hypertriglyceridemia) and deposition in the liver, and increased CO2 production.
How so? check out the metabolic pathway below to understand.
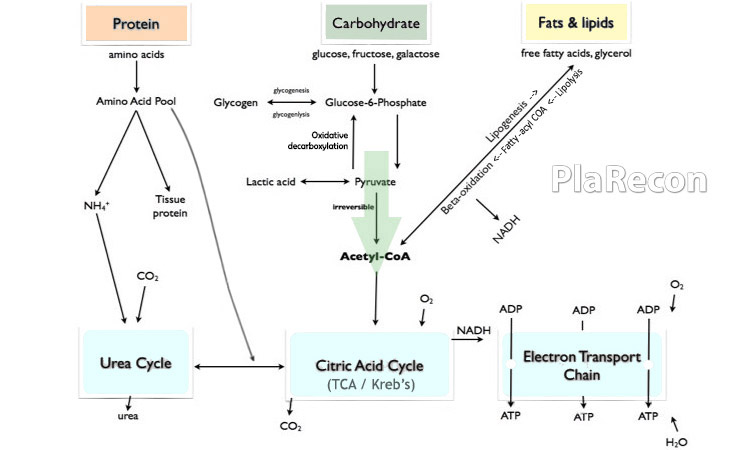
However, 50-60% of the overwhelming ‘caloric goal’ in severe burns paradoxically exceeds this limit of 5-7 g/kg/day that the body can metabolize and hence, the caloric requirement beyond that should be supplemented with an increase in protein intake.
The recommended rate of IV glucose administration is 5 – 7 mg/kg/min. [💡 Note: the units are correct] The catabolic hormones, due to burn stress also cause ‘relative insulin resistance’. Current recommendations are for “moderately strict” glucose control with a target of 110 – 150 mg/dl.
**A liter of 5% Dextrose (5D) contains 50 gm of dextrose or almost 200 kcal.
Fat (Lipids)
Lipids are needed for the production of free fatty acids (FFA), carriage of lipid-soluble vitamins involved in basic homeostasis and wound healing, and also provide supplementary calories. They should make up 3 – 15% of the ‘caloric goal’.
Fat in excess of 15% leads to the accumulation of free fatty acids in the liver leading to hepatic steatosis and hence, the percentage of dietary calories from fat should be limited and carefully monitored.
Nondietary fat calories, for example, 1% propofol solution has fat content equivalent to that in 10% intralipid emulsion, should also be taken into consideration.
Protein
Proteolysis is the hallmark of the hypermetabolic response to burns and protein synthesis is also twice that in uninjured. Protein supplement of 1.5 – 2 g/kg/day in adult and 2.5 – 4 g/kg/day in burned children balance the protein synthesis and breakdown due to burn hypermetabolism.
As mentioned above, there are limitations to the amount of carbohydrate and fat that can be used to meet the ‘caloric goal’ post-burn. The remaining energy requirement is to be met by proteins.
Agian, there are limits to the rate of effective protein metabolism as well. Protein intake above the values will lead to urea overload and azotemia, with limited additionally providing the substrate for wound healing and support of muscle mass.
Vitamins and Trace Minerals
Most burn centers administer a daily dose of a multivitamin supplement but there is no evidence-based recommendation currently.
Vitamin A prevents free radical damage, maintains immune function, and aids wound epithelialization while Vitamin C has antioxidant properties and has a critical role in wound healing (collagen cross-linking). Hence Vitamin A and C are supplemented (no evidence-based recommendation).
Trace minerals like Zinc, Selenium, and Copper are found to improve wound healing and immune functions] but there is no consensus on the use of the same currently.
Assessment of the Nutrition Support
Blood levels of transthyretin, C-reactive protein (CRP), Retinol-binding protein (RBP), transferrin, albumin have been assessed as a surrogate for nutritional status but none have not demonstrated any predictive capability and can be used in conjunction with other modalities. They may simply be markers of the severity of burns.
The elevated protein demands associated with burn hypermetabolism imply relevance for nitrogen balance monitoring in assessing nutritional adequacy. A negative nitrogen balance means excretion of nitrogen exceeds the daily intake (i.e., net catabolism), whereas a positive nitrogen balance is associated with muscle (lean body mass- LBM) gain.
Urinary urea nitrogen (UUN) has been demonstrated to be inaccurate. This unreliability may be primarily present in patients with exceptional hypercatabolic responses. Further, loss of protein through wound exudates complicates the calculation. In spite of these limitations, measurement of UUN and calculation of nitrogen balance on a weekly basis allows estimation of nitrogen catabolism and modification of protein goals based upon trends.
Indirect calorimetry (IDC) also provides insight into the substrate metabolism balance via the respiratory quotient (RQ = VCO2/ VO2= 0.7 to 1.0).
- RQ ≤ 0.7 implies that fat calories are increasingly utilized or underfeeding.
- RQ > 1.0 suggests lipogenesis from either high (>60%) carbohydrate intake or overfeeding.
Even IDC is affected by metabolic acid-base derangements, core temperature abnormalities, need for high levels of oxygen support, and hyper- or hypoventilation.
In the absence of superior monitoring methods, weekly IDC along with other means of monitoring nutritional status trends remains a cornerstone of nutrition management in burn patients.
Hence, with the heterogeneity of burn patients’ metabolic status and the associated variability in the findings from the abovementioned methods, employing a combination of clinical course, wound healing, objective measures and an overall focus on trends– optimizes monitoring
Complications
Complications of enteral feeding:
| Enteral Feeding | Causes |
|---|---|
| 1. Diarrhea –> dehydration –> hypernatraemia, hypokalemia, hypomagnesemia, constipation. | a) Osmotic effect of high glucose loads of tube feedings, b) Enteral medications such as antacids and antibiotics, c) Infectious causes- eg. pseudomembraneous colitis by C. diffcile. d) Overfeeding can also overwhelm the gut’s ability to absorb nutrients. |
| 2. Hyperglycemia | a) When >60% of the ‘calorie goal’ provided by carbohydrates, b) Relative insulin resistance due to catabolic hormones. |
| 3. Aspiration pneumonitis | a) Long-term supine position, b) Delayed gastric emptying, c) Altered mental status, d) Malpositioned feeding tube, e) Vomiting, f) Nonfunctional nasogastric drainage tube. |
| 4. Hypervolemic hyponatremia | a) Overhydration, b) Refeeding syndrome, c) Organ failure- liver, heart or kidney. |
| 5. Bowel necrosis | a) Occult abdominal compartment syndrome, b) Use of vasopressors, c) Bacterial overgrowth, d) Stagnation of tube feedings, bowel distension and ischemia caused by continued feeds in the setting of narcotic-induced ileus. Hence, enteral nutrition is not carefree and a close watch for subtle evidence of distension or distress is important. |
Further Reading
- Total Burn Care- Herndon, 5Ed (2018)
- Handbook of Burns- VOL 1,2 (Acute Burn Care, Rehabilitation, and Reconstruction) 1Ed (2012)
- Clinician’s Guide to Nutritional Therapy Following Major Burn Injury- Rollins (Clinics in Plastic Surgery- July 2017)
- Principles and Practice of Burn Care- Sujata Sarabahi- 1Ed (2009)

Tutorials & tips in Plastic Surgery
+ Weekly updates of high-quality webinars!